Difenilmetan Vidi još Reference Literatura Мени за навигацију101-81-5Interactive image7299100.002.708Diphenylmethane7580verifikuj„PubChem as a public resource for drug discovery.”2097051910.1016/j.drudis.2010.10.003уреди10.1016/S1574-1400(08)00012-1Organic ChemistryFundamentals of Organic Chemistry„Diphenylmethane”
Ароматични угљоводоници
organsko jedinjenjeformulommetanafenilbenzil hloridabenzenomLuisove kiselinealuminijum trihlorid
(function()var node=document.getElementById("mw-dismissablenotice-anonplace");if(node)node.outerHTML="u003Cdiv class="mw-dismissable-notice"u003Eu003Cdiv class="mw-dismissable-notice-close"u003E[u003Ca tabindex="0" role="button"u003Eсакријu003C/au003E]u003C/divu003Eu003Cdiv class="mw-dismissable-notice-body"u003Eu003Cdiv id="localNotice" lang="sr" dir="ltr"u003Eu003Cdiv style="position:relative; overflow:hidden; background-color:#5E9DC8; text-align:center; color:white; font-size:1.25em; font-weight:bold; line-height:1.5em; margin-top: 5px;"u003Eu003Cuu003Eu003Ca href="/wiki/%D0%92%D0%B8%D0%BA%D0%B8%D0%BF%D0%B5%D0%B4%D0%B8%D1%98%D0%B0:%D0%A2%D0%B0%D0%BA%D0%BC%D0%B8%D1%87%D0%B5%D1%9A%D0%B5_%D1%83_%D0%BF%D0%B8%D1%81%D0%B0%D1%9A%D1%83_%D1%87%D0%BB%D0%B0%D0%BD%D0%B0%D0%BA%D0%B0/%D0%A6%D0%95%D0%95_%D0%BF%D1%80%D0%BE%D0%BB%D0%B5%D1%9B%D0%B5_2019" title="Википедија:Такмичење у писању чланака/ЦЕЕ пролеће 2019"u003Eu003Cspan style="color:white"u003EТакмичите се у писању чланака о Средњој и Источној Европи од 21. марта до 31. маја!u003C/spanu003Eu003C/au003Eu003C/uu003Eu003C/divu003Enu003Cdiv style="position:relative; overflow:hidden; background-color:#5E9DC8; text-align:center; color:white; font-size:1.25em; font-weight:bold; line-height:1.5em; margin-top: 5px;"u003Eu003Cuu003Eu003Ca href="/wiki/%D0%92%D0%B8%D0%BA%D0%B8%D0%BF%D0%B5%D0%B4%D0%B8%D1%98%D0%B0:%D0%93%D0%BB%D0%B0%D1%81%D0%B0%D1%9A%D0%B5/%D0%94%D0%BE%D0%BF%D1%83%D0%BD%D0%B0_%D0%BF%D1%80%D0%B0%D0%B2%D0%B8%D0%BB%D0%B0_%D0%BE_%D0%B8%D0%B7%D0%B0%D0%B1%D1%80%D0%B0%D0%BD%D0%B8%D0%BC_%D1%81%D0%BB%D0%B8%D0%BA%D0%B0%D0%BC%D0%B0" title="Википедија:Гласање/Допуна правила о изабраним сликама"u003Eu003Cspan style="color:white"u003EУ току је гласање о допуни правила везаних за изабране слике!u003C/spanu003Eu003C/au003Eu003C/uu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003E";());
Difenilmetan
Иди на навигацију
Иди на претрагу
 | |
 | |
| Nazivi | |
|---|---|
IUPAC naziv 1,1'-methanediyldibenzene | |
| Drugi nazivi benzylbenzene | |
| Identifikacija | |
CAS broj |
|
3D model (Jmol) |
|
ChemSpider |
|
ECHA InfoCard | 100.002.708 |
MeSH | Diphenylmethane |
PubChem[1][2]CID |
|
SMILES
| |
| Svojstva | |
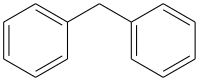
Hemijska formula | C13H12 |
Molarna masa | 168.234 |
Agregatno stanje | bezbojno ulje |
Gustina | 1.006 g/mL |
Tačka topljenja | 22-24 °C |
Tačka ključanja | 264 °C |
Rastvorljivost u vodi | nepolarni organski rastvarač |
| Opasnosti | |
| Opasnost u toku rada | zapaljiv |
Tačka paljenja | >230 °F |
| Srodna jedinjenja | |
Srodna jedinjenja | Difenilmetanol |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |
Reference infokutije | |
Difenilmetan je organsko jedinjenje sa formulom (C6H5)2CH2.[3][4] Ovo jedinjenje se sastoji od metana kod koga su dva atoma vodonika zamenjena fenil grupama. Difenilmetan je često korišteni skeleton u organskoj hemiji. Difenilmetilna grupa je poznata kao benzhidril.
On se priprema reakcijom benzil hlorida sa benzenom u prisustvu Luisove kiseline kao što je aluminijum trihlorid:[5]
- C6H5CH2Cl + C6H6 → (C6H5)2CH2 + HCl
Vidi još
- Benzhidrilna jedinjenja
Reference
^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (I изд.). Oxford University Press. ISBN 978-0-19-850346-0.
^ McMurry John E. (1992). Fundamentals of Organic Chemistry (3rd изд.). Belmont: Wadsworth. ISBN 0-534-16218-5.
^ W. W. Hartman and Ross Phillips (1943). „Diphenylmethane”. Org. Synth. ; Coll. Vol., 2, стр. 232
Literatura
Категорија:
- Ароматични угљоводоници
(RLQ=window.RLQ||[]).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.592","walltime":"0.824","ppvisitednodes":"value":5121,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":73052,"limit":2097152,"templateargumentsize":"value":11367,"limit":2097152,"expansiondepth":"value":19,"limit":40,"expensivefunctioncount":"value":0,"limit":500,"unstrip-depth":"value":0,"limit":20,"unstrip-size":"value":6926,"limit":5000000,"entityaccesscount":"value":1,"limit":400,"timingprofile":["100.00% 777.479 1 -total"," 78.12% 607.373 1 Шаблон:Chembox-lat"," 42.17% 327.886 1 Шаблон:Chembox_Identifiers-lat"," 32.48% 252.561 5 Шаблон:Chembox_headerbar"," 31.94% 248.340 15 Шаблон:Trim"," 21.44% 166.685 1 Шаблон:Reflist"," 16.41% 127.573 7 Шаблон:Main_other"," 15.83% 123.103 1 Шаблон:Chembox_parametercheck"," 13.14% 102.157 4 Шаблон:Chembox_image"," 12.80% 99.481 2 Шаблон:Chembox_image_cell"],"scribunto":"limitreport-timeusage":"value":"0.238","limit":"10.000","limitreport-memusage":"value":3921580,"limit":52428800,"cachereport":"origin":"mw1306","timestamp":"20190512214746","ttl":2592000,"transientcontent":false););"@context":"https://schema.org","@type":"Article","name":"Difenilmetan","url":"https://sr.wikipedia.org/wiki/Difenilmetan","sameAs":"http://www.wikidata.org/entity/Q412882","mainEntity":"http://www.wikidata.org/entity/Q412882","author":"@type":"Organization","name":"u0421u0430u0440u0430u0434u043du0438u0446u0438 u043fu0440u043eu0458u0435u043au0430u0442u0430 u0412u0438u043au0438u043cu0435u0434u0438u0458u0435","publisher":"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png","datePublished":"2011-04-19T13:25:35Z","dateModified":"2017-01-04T03:43:36Z","image":"https://upload.wikimedia.org/wikipedia/commons/0/06/Diphenylmethane2.png"(RLQ=window.RLQ||[]).push(function()mw.config.set("wgBackendResponseTime":214,"wgHostname":"mw1325"););