Heptabarb Садржај Osobine Reference Literatura Spoljašnje veze Мени за навигацију509-86-4Interactive imageCHEBI:58807410081DB01354100.007.371C1772510518verifikuj„Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H„PubChem as a public resource for drug discovery.”2097051910.1016/j.drudis.2010.10.003уреди10.1016/S1574-1400(08)00012-1„DrugBank 3.0: a comprehensive resource for omics research on drugs”30137092105968210.1093/nar/gkq1126уреди„DrugBank: a knowledgebase for drugs, drug actions and drug targets”22388891804841210.1093/nar/gkm958уреди„Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”10.1021/jp980230o„Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”1174957310.1021/ci000392tуреди„Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”1102028610.1021/jm000942eуредиOrganic ChemistryAdvanced Organic Chemistry: Reactions, Mechanisms, and StructureHandbook of Heterocyclic ChemistryHeptabarbVikimedijinoj ostaviHeptabarbital
СедативиБарбитуратиАцетамиди
organsko jedinjenjeatomaugljenikamolekulsku masuDa
(function()var node=document.getElementById("mw-dismissablenotice-anonplace");if(node)node.outerHTML="u003Cdiv class="mw-dismissable-notice"u003Eu003Cdiv class="mw-dismissable-notice-close"u003E[u003Ca tabindex="0" role="button"u003Eсакријu003C/au003E]u003C/divu003Eu003Cdiv class="mw-dismissable-notice-body"u003Eu003Cdiv id="localNotice" lang="sr" dir="ltr"u003Eu003Cdiv style="position:relative; overflow:hidden; background-color:#5E9DC8; text-align:center; color:white; font-size:1.25em; font-weight:bold; line-height:1.5em; margin-top: 5px;"u003Eu003Cuu003Eu003Ca href="/wiki/%D0%92%D0%B8%D0%BA%D0%B8%D0%BF%D0%B5%D0%B4%D0%B8%D1%98%D0%B0:%D0%A2%D0%B0%D0%BA%D0%BC%D0%B8%D1%87%D0%B5%D1%9A%D0%B5_%D1%83_%D0%BF%D0%B8%D1%81%D0%B0%D1%9A%D1%83_%D1%87%D0%BB%D0%B0%D0%BD%D0%B0%D0%BA%D0%B0/%D0%A6%D0%95%D0%95_%D0%BF%D1%80%D0%BE%D0%BB%D0%B5%D1%9B%D0%B5_2019" title="Википедија:Такмичење у писању чланака/ЦЕЕ пролеће 2019"u003Eu003Cspan style="color:white"u003EТакмичите се у писању чланака о Средњој и Источној Европи од 21. марта до 31. маја!u003C/spanu003Eu003C/au003Eu003C/uu003Eu003C/divu003Enu003Cdiv style="position:relative; overflow:hidden; background-color:#5E9DC8; text-align:center; color:white; font-size:1.25em; font-weight:bold; line-height:1.5em; margin-top: 5px;"u003Eu003Cuu003Eu003Ca href="/wiki/%D0%92%D0%B8%D0%BA%D0%B8%D0%BF%D0%B5%D0%B4%D0%B8%D1%98%D0%B0:%D0%93%D0%BB%D0%B0%D1%81%D0%B0%D1%9A%D0%B5/%D0%94%D0%BE%D0%BF%D1%83%D0%BD%D0%B0_%D0%BF%D1%80%D0%B0%D0%B2%D0%B8%D0%BB%D0%B0_%D0%BE_%D0%B8%D0%B7%D0%B0%D0%B1%D1%80%D0%B0%D0%BD%D0%B8%D0%BC_%D1%81%D0%BB%D0%B8%D0%BA%D0%B0%D0%BC%D0%B0" title="Википедија:Гласање/Допуна правила о изабраним сликама"u003Eu003Cspan style="color:white"u003EУ току је гласање о допуни правила везаних за изабране слике!u003C/spanu003Eu003C/au003Eu003C/uu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003E";());
Heptabarb
(преусмерено са Heptabarbital)
Иди на навигацију
Иди на претрагу
 | |
| Identifikacija | |
|---|---|
CAS broj |
|
3D model (Jmol) |
|
ChEBI |
|
ChemSpider |
|
DrugBank |
|
ECHA InfoCard | 100.007.371 |
KEGG[1] |
|
PubChem[2][3]CID |
|
SMILES
| |
| Svojstva | |
Hemijska formula | C13H18N2O3 |
Molarna masa | 250,294 |
Tačka topljenja | 174 |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |
Reference infokutije | |
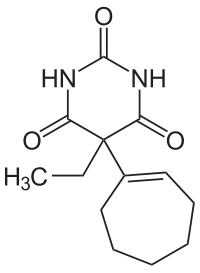
Heptabarb je organsko jedinjenje, koje sadrži 13 atoma ugljenika i ima molekulsku masu od 250,294 Da.[4][5]
Садржај
1 Osobine
2 Reference
3 Literatura
4 Spoljašnje veze
Osobine
| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 3 |
| Broj donora vodonika | 2 |
| Broj rotacionih veza | 2 |
Particioni koeficijent[6] (ALogP) | 2,2 |
Rastvorljivost[7] (logS, log(mol/L)) | -3,0 |
Polarna površina[8] (PSA, Å2) | 75,3 |
Reference
^ Joanne Wixon; Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast. 17 (1): 48—55. doi:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
^ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035—41. PMC 3013709 . PMID 21059682. doi:10.1093/nar/gkq1126.
. PMID 21059682. doi:10.1093/nar/gkq1126.
^ David S. Wishart; Craig Knox; An Chi Guo; Dean Cheng; Savita Shrivastava; Dan Tzur; Bijaya Gautam; Murtaza Hassanali (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic acids research. 36 (Database issue): D901—6. PMC 2238889 . PMID 18048412. doi:10.1093/nar/gkm958.
. PMID 18048412. doi:10.1093/nar/gkm958.
^ Ghose, A.K.; Viswanadhan V.N. & Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A. 102: 3762—3772. doi:10.1021/jp980230o.
^ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488—1493. PMID 11749573. doi:10.1021/ci000392t.
^ Ertl P.; Rohde B.; Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714—3717. PMID 11020286. doi:10.1021/jm000942e.
Literatura
.mw-parser-output .refbeginfont-size:90%;margin-bottom:0.5em.mw-parser-output .refbegin-hanging-indents>ullist-style-type:none;margin-left:0.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>ddmargin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none.mw-parser-output .refbegin-100font-size:100%
Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (I изд.). Oxford University Press. ISBN 978-0-19-850346-0.
Smith, Michael B.; March, Jerry (2007). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th изд.). New York: Wiley-Interscience. ISBN 0-471-72091-7.
Katritzky A.R.; Pozharskii A.F. (2000). Handbook of Heterocyclic Chemistry (Second изд.). Academic Press. ISBN 0080429882.
Spoljašnje veze
Heptabarb na Vikimedijinoj ostavi. |
- Heptabarbital
| Molimo Vas, obratite pažnju na važno upozorenje u vezi sa temama iz oblasti medicine (zdravlja). |
Категорије:
- Седативи
- Барбитурати
- Ацетамиди
(RLQ=window.RLQ||[]).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.768","walltime":"0.992","ppvisitednodes":"value":5403,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":114842,"limit":2097152,"templateargumentsize":"value":12443,"limit":2097152,"expansiondepth":"value":19,"limit":40,"expensivefunctioncount":"value":0,"limit":500,"unstrip-depth":"value":0,"limit":20,"unstrip-size":"value":15745,"limit":5000000,"entityaccesscount":"value":1,"limit":400,"timingprofile":["100.00% 914.024 1 -total"," 59.96% 548.032 1 Шаблон:Chembox-lat"," 42.88% 391.950 1 Шаблон:Chembox_Identifiers-lat"," 30.60% 279.673 2 Шаблон:Chembox_headerbar"," 30.28% 276.743 6 Шаблон:Trim"," 25.85% 236.280 1 Шаблон:Reflist"," 20.21% 184.724 8 Шаблон:Cite_journal"," 16.96% 155.008 4 Шаблон:Main_other"," 16.64% 152.116 1 Шаблон:Chembox_parametercheck"," 12.59% 115.092 5 Шаблон:Cite_pmid"],"scribunto":"limitreport-timeusage":"value":"0.380","limit":"10.000","limitreport-memusage":"value":5560583,"limit":52428800,"cachereport":"origin":"mw1309","timestamp":"20190512215236","ttl":2592000,"transientcontent":false););"@context":"https://schema.org","@type":"Article","name":"Heptabarb","url":"https://sr.wikipedia.org/wiki/Heptabarb","sameAs":"http://www.wikidata.org/entity/Q410829","mainEntity":"http://www.wikidata.org/entity/Q410829","author":"@type":"Organization","name":"u0421u0430u0440u0430u0434u043du0438u0446u0438 u043fu0440u043eu0458u0435u043au0430u0442u0430 u0412u0438u043au0438u043cu0435u0434u0438u0458u0435","publisher":"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png","datePublished":"2013-12-17T03:37:05Z","dateModified":"2014-01-13T04:10:59Z","image":"https://upload.wikimedia.org/wikipedia/commons/9/96/Heptabarbital.svg"(RLQ=window.RLQ||[]).push(function()mw.config.set("wgBackendResponseTime":137,"wgHostname":"mw1265"););