Rakloprid Reference Spoljašnje veze Мени за навигацију84225-95-63033769942298373430K3SOZ7GCHEMBL8809401567410.1016/0006-2952(85)90778-6902465610.1038/385590a0ChemSpiderу
Типични антипсихотициДопамински антагонистиБензамидиПиролидиниФенолни етриХлороарениАмиди
antagonistdopaminskom receptoruradioizotopompozitronskom emisiono tomografskomdopaminskogD2 dopaminskireceptor
(function()var node=document.getElementById("mw-dismissablenotice-anonplace");if(node)node.outerHTML="u003Cdiv class="mw-dismissable-notice"u003Eu003Cdiv class="mw-dismissable-notice-close"u003E[u003Ca tabindex="0" role="button"u003Eсакријu003C/au003E]u003C/divu003Eu003Cdiv class="mw-dismissable-notice-body"u003Eu003Cdiv id="localNotice" lang="sr" dir="ltr"u003Eu003Cdiv class="noticebanner"u003Eu003Cdiv class="plainlinks" style="background-image: -moz-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: -o-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: -webkit-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: linear-gradient(to bottom, #fefefe, #fefefe, #f0f0f0);; -moz-border-radius: 10px; -webkit-border-radius: 10px; border-radius:10px; margin-top:10px; position:relative; height:25px; border-style:solid; border-width:1px; color:#aaa; border-color:u0026#32;; border:; font-family: u0026#39;Helveticau0026#39;, u0026#39;Arialu0026#39;, sans-serif; line-height: 18px; background-color:; overflow:hidden;"u003Eu003Cdiv style="display:block; position: absolute; top:4px; width:100%; text-align:center;;"u003Eu003Cdiv style="font-weight:bold; color:#000085; font-size:14px; line-height:25px"u003Eu003Cdiv style="padding-left:50px;"u003Eu003C/divu003Eu003C/divu003Eu003Cdiv style="margin-top: 2px; font-weight:normal; color:#555; font-size:15px; line-height:15px;"u003Eu003Cdiv style="padding-left:50px;"u003EУчествуј у u003Cbu003Eu003Ca href="https://meta.wikimedia.org/wiki/%D0%92%D0%B5%D0%BB%D0%B8%D0%BA%D0%BE_%D0%BF%D1%80%D0%BE%D0%BB%D0%B5%D1%9B%D0%BD%D0%BE_%D1%81%D1%80%D0%B5%D1%92%D0%B8%D0%B2%D0%B0%D1%9A%D0%B5_%D0%9E%D1%81%D1%82%D0%B0%D0%B2%D0%B5" class="extiw" title="meta:Велико пролећно сређивање Оставе"u003EВеликом пролећном сређивању Оставеu003C/au003Eu003C/bu003E од 15. марта до 15. априла!u003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Enu003Cdiv class="noticebanner"u003Eu003Cdiv class="plainlinks" style="background-image: -moz-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: -o-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: -webkit-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: linear-gradient(to bottom, #fefefe, #fefefe, #f0f0f0);; -moz-border-radius: 10px; -webkit-border-radius: 10px; border-radius:10px; margin-top:10px; position:relative; height:25px; border-style:solid; border-width:1px; color:#aaa; border-color:u0026#32;; border:; font-family: u0026#39;Helveticau0026#39;, u0026#39;Arialu0026#39;, sans-serif; line-height: 18px; background-color:; overflow:hidden;"u003Eu003Cdiv style="display:block; position: absolute; top:4px; width:100%; text-align:center;;"u003Eu003Cdiv style="font-weight:bold; color:#000085; font-size:14px; line-height:25px"u003Eu003Cdiv style="padding-left:50px;"u003Eu003C/divu003Eu003C/divu003Eu003Cdiv style="margin-top: 2px; font-weight:normal; color:#555; font-size:15px; line-height:15px;"u003Eu003Cdiv style="padding-left:50px;"u003EТакмичи се у писању u003Cbu003Eu003Ca href="/wiki/%D0%92%D0%B8%D0%BA%D0%B8%D0%BF%D0%B5%D0%B4%D0%B8%D1%98%D0%B0:%D0%A2%D0%B0%D0%BA%D0%BC%D0%B8%D1%87%D0%B5%D1%9A%D0%B5_%D1%83_%D0%BF%D0%B8%D1%81%D0%B0%D1%9A%D1%83_%D1%87%D0%BB%D0%B0%D0%BD%D0%B0%D0%BA%D0%B0/%D0%A6%D0%95%D0%95_%D0%BF%D1%80%D0%BE%D0%BB%D0%B5%D1%9B%D0%B5_2019" title="Википедија:Такмичење у писању чланака/ЦЕЕ пролеће 2019"u003Eчланака о средњој и источној Европиu003C/au003Eu003C/bu003E од 21. марта до 31. маја!u003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Enu003Cdiv class="noticebanner"u003Eu003Cdiv class="plainlinks" style="background-image: -moz-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: -o-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: -webkit-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: linear-gradient(to bottom, #fefefe, #fefefe, #f0f0f0);; -moz-border-radius: 10px; -webkit-border-radius: 10px; border-radius:10px; margin-top:10px; position:relative; height:25px; border-style:solid; border-width:1px; color:#aaa; border-color:u0026#32;; border:; font-family: u0026#39;Helveticau0026#39;, u0026#39;Arialu0026#39;, sans-serif; line-height: 18px; background-color:; overflow:hidden;"u003Eu003Cdiv style="display:block; position: absolute; top:4px; width:100%; text-align:center;;"u003Eu003Cdiv style="font-weight:bold; color:#000085; font-size:14px; line-height:25px"u003Eu003Cdiv style="padding-left:50px;"u003Eu003C/divu003Eu003C/divu003Eu003Cdiv style="margin-top: 2px; font-weight:normal; color:#555; font-size:15px; line-height:15px;"u003Eu003Cdiv style="padding-left:50px;"u003EУ току је расправа о u003Cbu003Eu003Ca href="/wiki/%D0%92%D0%B8%D0%BA%D0%B8%D0%BF%D0%B5%D0%B4%D0%B8%D1%98%D0%B0:%D0%93%D0%BB%D0%B0%D1%81%D0%B0%D1%9A%D0%B5/%D0%9F%D1%80%D0%B5%D0%B4%D0%BB%D0%BE%D0%B3/%D0%9F%D1%80%D0%B0%D0%B2%D0%BE_%D0%B0%D0%B4%D0%BC%D0%B8%D0%BD%D0%B8%D1%81%D1%82%D1%80%D0%B0%D1%82%D0%BE%D1%80_%D0%B8%D0%BD%D1%82%D0%B5%D1%80%D1%84%D0%B5%D1%98%D1%81%D0%B0" title="Википедија:Гласање/Предлог/Право администратор интерфејса"u003Eадминистраторима интерфејсаu003C/au003Eu003C/bu003E.u003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Enu003Cdiv class="noticebanner"u003Eu003Cdiv class="plainlinks" style="background-image: -moz-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: -o-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: -webkit-linear-gradient(top, #fefefe, #fefefe, #f0f0f0); background-image: linear-gradient(to bottom, #fefefe, #fefefe, #f0f0f0);; -moz-border-radius: 10px; -webkit-border-radius: 10px; border-radius:10px; margin-top:10px; position:relative; height:25px; border-style:solid; border-width:1px; color:#aaa; border-color:u0026#32;; border:; font-family: u0026#39;Helveticau0026#39;, u0026#39;Arialu0026#39;, sans-serif; line-height: 18px; background-color:; overflow:hidden;"u003Eu003Cdiv style="display:block; position: absolute; top:4px; width:100%; text-align:center;;"u003Eu003Cdiv style="font-weight:bold; color:#000085; font-size:14px; line-height:25px"u003Eu003Cdiv style="padding-left:50px;"u003Eu003C/divu003Eu003C/divu003Eu003Cdiv style="margin-top: 2px; font-weight:normal; color:#555; font-size:15px; line-height:15px;"u003Eu003Cdiv style="padding-left:50px;"u003EУ току су гласања о u003Cbu003Eu003Ca href="/wiki/%D0%92%D0%B8%D0%BA%D0%B8%D0%BF%D0%B5%D0%B4%D0%B8%D1%98%D0%B0:%D0%93%D0%BB%D0%B0%D1%81%D0%B0%D1%9A%D0%B5/%D0%9F%D0%BE%D0%B2%D0%B5%D1%9B%D0%B0%D1%9A%D0%B5_%D0%BF%D1%80%D0%BE%D1%81%D1%82%D0%BE%D1%80%D0%B0_%D0%B7%D0%B0_%D1%81%D0%BB%D0%B8%D0%BA%D0%B5_%D0%BD%D0%B0_%D0%B3%D0%BB%D0%B0%D0%B2%D0%BD%D0%BE%D1%98_%D1%81%D1%82%D1%80%D0%B0%D0%BD%D0%B8" title="Википедија:Гласање/Повећање простора за слике на главној страни"u003Eповећању простора за слике на Главној страниu003C/au003Eu003C/bu003E и u003Cbu003Eu003Ca href="/wiki/%D0%92%D0%B8%D0%BA%D0%B8%D0%BF%D0%B5%D0%B4%D0%B8%D1%98%D0%B0:%D0%93%D0%BB%D0%B0%D1%81%D0%B0%D1%9A%D0%B5/%D0%9F%D1%80%D0%B0%D0%B2%D0%B8%D0%BB%D0%B0_%D0%B7%D0%B0_%D0%BA%D0%BE%D1%80%D0%B8%D1%88%D1%9B%D0%B5%D1%9A%D0%B5_%D0%B8%D0%BC%D0%B5%D0%BD%D1%81%D0%BA%D0%BE%D0%B3_%D0%BF%D1%80%D0%BE%D1%81%D1%82%D0%BE%D1%80%D0%B0_%D0%9D%D0%B0%D1%86%D1%80%D1%82" title="Википедија:Гласање/Правила за коришћење именског простора Нацрт"u003Eкоришћењу именског простора Нацртu003C/au003Eu003C/bu003E.u003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003E";());
Rakloprid
Иди на навигацију
Иди на претрагу
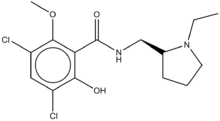 | |
IUPAC ime | |
|---|---|
3,5-dihlor-N[(2S)-1-etilpirolidin-2-il]metil2-hidroksi-6-metoksibenzamid | |
Farmakokinetički podaci | |
| Poluvreme eliminacije | 30 min |
| Identifikatori | |
| CAS broj | 84225-95-6 |
| ATC kod | none |
| PubChem | CID 3033769 |
| IUPHAR/BPS | 94 |
| ChemSpider | 2298373 |
| UNII | 430K3SOZ7G |
| ChEMBL | CHEMBL8809 |
| Hemijski podaci | |
| Formula | C15H20Cl2N2O3 |
| Molarna masa | 347,236 g/mol |
SMILES
| |
InChI
| |
Rakloprid je sintetičko jedinjenje koje deluje kao antagonist na D2 dopaminskom receptoru.[1]
On se može radio obeležiti radioizotopom ugljenik-11 i koristiti u pozitronskom emisiono tomografskom (PET) skeniranju za utvrđivanje stepena dopaminskog vezivanja za D2 dopaminski receptor. Na primer, jedna studija je utvrdila da postoji umanjeno vezivanje kod osoba sa emocionom otuđenošću.[2]
Reference
^ C. Kohler; H. Hall; S. O. Ogren; L. Gawell (1985). „Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain”. Biochemical Pharmacology. 34 (13): 2251—2259. PMID 4015674. doi:10.1016/0006-2952(85)90778-6.
^ Lars Farde; J. Petter Gustavsson; Erik Jönsson (1997). „D2 dopamine receptors and personality traits”. Nature. 385 (6617): 590. PMID 9024656. doi:10.1038/385590a0.
Spoljašnje veze
- ChemSpider
| Molimo Vas, obratite pažnju na važno upozorenje u vezi sa temama iz oblasti medicine (zdravlja). |
Категорије:
- Типични антипсихотици
- Допамински антагонисти
- Бензамиди
- Пиролидини
- Фенолни етри
- Хлороарени
- Амиди
(window.RLQ=window.RLQ||[]).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.528","walltime":"0.655","ppvisitednodes":"value":4358,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":247179,"limit":2097152,"templateargumentsize":"value":27432,"limit":2097152,"expansiondepth":"value":17,"limit":40,"expensivefunctioncount":"value":0,"limit":500,"unstrip-depth":"value":0,"limit":20,"unstrip-size":"value":2764,"limit":5000000,"entityaccesscount":"value":0,"limit":400,"timingprofile":["100.00% 492.760 1 -total"," 53.28% 262.528 1 Шаблон:Drugbox-lat"," 48.84% 240.656 3 Шаблон:Infobox"," 23.42% 115.424 1 Шаблон:Dopaminergici-lat"," 22.05% 108.652 1 Шаблон:Navbox_with_collapsible_sections"," 15.10% 74.386 1 Шаблон:Reflist"," 14.41% 70.983 8 Шаблон:Navbox_subgroup"," 12.15% 59.894 2 Шаблон:Cite_journal"," 6.02% 29.681 1 Шаблон:Infobox_drug-lat/chemical_formula"," 3.83% 18.886 2 Шаблон:Keypress"],"scribunto":"limitreport-timeusage":"value":"0.152","limit":"10.000","limitreport-memusage":"value":3825738,"limit":52428800,"cachereport":"origin":"mw1330","timestamp":"20190403005839","ttl":2592000,"transientcontent":false););"@context":"https://schema.org","@type":"Article","name":"Rakloprid","url":"https://sr.wikipedia.org/wiki/Rakloprid","sameAs":"http://www.wikidata.org/entity/Q7279814","mainEntity":"http://www.wikidata.org/entity/Q7279814","author":"@type":"Organization","name":"u0421u0430u0440u0430u0434u043du0438u0446u0438 u043fu0440u043eu0458u0435u043au0430u0442u0430 u0412u0438u043au0438u043cu0435u0434u0438u0458u0435","publisher":"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png","datePublished":"2012-03-23T05:22:08Z","dateModified":"2017-11-15T02:04:37Z","image":"https://upload.wikimedia.org/wikipedia/commons/3/3b/Raclopride.png"(window.RLQ=window.RLQ||[]).push(function()mw.config.set("wgBackendResponseTime":135,"wgHostname":"mw1266"););